Get Marking Schemes From Here
Answer all the questions in the spaces provided.
- The set up below can be used to prepare oxygen gas. Study it and answer the questions that
follow
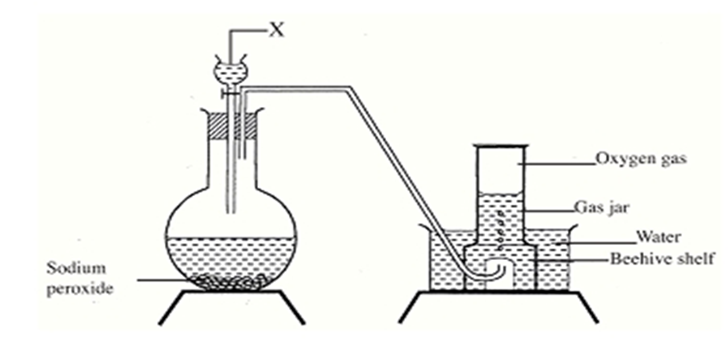
- Identify substance X.1mk
- What property of oxygen makes it possible for it to be collected as shown in the above
set up? 1mk
- State two uses of oxygen. 2kms
- Describe an experimental procedure that can be used to extract oil from nut seeds.3mks
- In terms of structure and bonding, explain the following observations:
- The melting point of water H2O is higher than that of H2S. 2mks
- Melting point of chlorine is lower than that of sulphur. 2mks
- a. Define the term salt. 1mk
b.Apart from direct synthesis name two other methods of salt preparation. 2mks
c.The diagram below illustrates a method of preparing salts by direct synthesis.

d.This method can be used to prepare either aluminum chloride or iron (III) chloride.
Explain. 1mk
e.What is the purpose of anhydrous calcium chloride? 1mk
- Define the following terms. 3mks
- Normal salt
- Efflorescent salts
- Deliquescent salts
- a. What is meant by an allotrope? 1mk
b.Give two allotropes of carbon. 2mks
- The grid below is part of the periodic table. Use it answer the questions that follow. (The letters are not the actual symbols of the elements).
- Which is the most reactive non-metallic element shown in the table? Explain. (2 mks)
- (i) Write the formula of the compound formed when element A reacts with element B. (1 mk)
(ii) Name the bond type in the compound formed in b (i) above. (1 mk)
- (i) What is the name given to the group of elements where C, G and H belong? (1 mk)
- The melting points of elements F and G are 1410 0C and -101 0C respectively. In terms of structure and bonding, explain why there is a large difference in the melting points of F and G. (2 marks)
- D forms two oxides. Write the formula of each of two oxides. (1 mark)
- J is an element that belongs to the 3rd period of the periodic table and is a member of the alkaline earth elements. Show the position of J in the grid. (1 mark)
- i) Define the term isotope.1mk
ii) The table below gives the number of electrons, protons and neutrons in substances X, Y and Z Study it and answers the questions that follow.
| Substance | Electrons | Protons | Neutrons |
| X | 10 | 10 8 | 10 10 |
| Y | 10 | ||
| z | 8 | 8 | 8 |
- Which letter represents an ion? (1 mark)
- Which of the substances are isotopes? Give a reason. (2 marks
- Show the electron arrangement of Y 1mk
- a. What is an impurity? 1mk
b .The curve below represents the variation of temperature with time when pure and
Impure samples of a solid were heated separately.

Which curve sows the variation in temperature for the pure solid? Explain.2mks
- The diagram below shows the physical state of matter. Study it and answer the questions that follow.
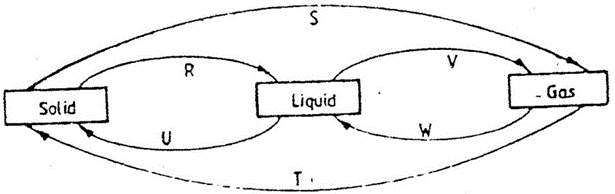
- Identify the processes R, V, w and U. 4mks
R
V
W
U
- Name the process S and T. 1mk
- Name two substances which can undergo the process represented by S and T. 2mks
- Define the term ionization energy. 1mk
- The table below gives the energy required to remove the outermost electron for some group I elements.
| Element | I | II | III | IV |
| Ionization Energy kJmol-1 | 494 | 418 | 519 | 376 |
Arrange the elements in order of their reactivity starting with the most reactive. 2mks
- The table below shows some properties of substances C,D and E. Study it and answer the questions that follow.
| Substance | M.P (0C) | Solubility in water | Electrical solid state | Conductivity molten stated |
| E | -39 | Insoluble | Good | good |
| D | 1610 | Insoluble | Poor | poor |
| E | 801 | Soluble | Poor | good |
Select substance
- With a giant molecular structure. 1mk
- That has ionic bonding. 1mk
- That is a metal. 1mk
(1mk)
- An element Y has the electronic configuration 2.8.5
- Which period and group of the periodic table does the element belong? 2mks
- Group
- Period
- Write electron arrangement of the ion of Y. 1mk
c) Explain the difference between the atomic radium of element Y and
its ionic radius. 2mks
- The table below shows the PH values of solutions I, II, III and IV.
| Solution | I | II | III | IV |
| PH | 2 | 7 | 11 | 14 |
- Which solution is likely to be that of: 3mks
- Calcium hydroxide
- Sodium chloride
- Potassium hydroxide
- a. What is the chemical name for rust? 1mk
b.State three conditions necessary for rusting. 3mks
c. State tree methods of preventing rusting. 3mks
- Write an equation to show the effect of heat on: 3mks
- NaNO3
- Zn (NO3)2
- AgNO3