
1. A. In an experiment to determine the percentage of oxygen in air, the apparatus below were set up. Study the set up and the information provided to answer the
questions that follow.
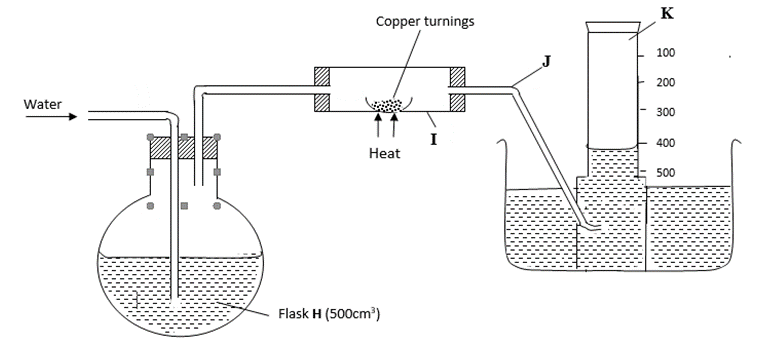
A 500cm3 measuring cylinder K was filled with water and assembled for gas collection. Copper turnings were heated red hot and water was slowly passed into 500cm3 flask H until it reached the 500cm3 mark. A colourless gas was collected in K.
(i) What was the purpose of passing water into flask H? (1 mark)
……………………………………………………………………………………………………………………………
(ii) What observations were made in the tube I? (1 mark)
……………………………………………………………………………………………………………………………
(iii) Name one of the gases that is likely to be found in J. (1 mark)
……………………………………………………………………………………………………………………………
(iv) What was the volume of the gas collected in the measuring cylinder at the end of the experiment? (1 mark)
……………………………………………………………………………………………………………………………
(v) Calculate the percentage of oxygen in air using the above results. (2 marks)
B. Study the diagram below and answer the questions that follow.

(a) Give one observation made in the combustion tube after some time. (1 mark)
……………………………………………………………………………………………………………………………
(b) Write an equation for the formation of the colourless liquid Y. (1 mark)
……………………………………………………………………………………………………………………………
(c) What was the aim of the above experiment as demonstrated in the combustion
tube? Explain. (2 marks)
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
2. Use the information below to answer the questions that follow. The letters are not the actual symbols of the elements.
| Element | Atomic No. | M.P0C | B.P0C | Ionic radius (nm) |
| P | 11 | 98 | 890 | 0.095 |
| Q | 12 | 650 | 1110 | 0.065 |
| R | 13 | 660 | 2470 | 0.050 |
| S | 14 | 1410 | 2360 | 0.041 |
| T | 15 | 44.2 & 590 | 280 | 0.034 |
| U | 16 | 113 & 119 | 445 | 0.184 |
| V | 17 | -101 | -35 | 0.181 |
| W | 18 | -189 | -186 | – |
(a) (i) Write the electronic configuration of the atoms represented by letters T and W. (1 mark)
(ii) State the nature of the oxides of the elements represented by Q and U. (2 marks)
……………………………………………………………………………………………………………….
……………………………………………………………………………………………………………….
(b) Why does the elements represented by the letters T and U have two values of melting points? (1 mark)
……………………………………………………………………………………………………………………………
(c) Explain the following observations in terms of structure and bonding.
(i) There is an increase in boiling point from P to R. (2 marks)
……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(ii) Element S has a high boiling point. (2 marks)
……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(iii) There is a decrease in boiling points from U to W. (2 marks)
……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
(d) (i) Compare the atomic radius of U and V. (1 mark)
…………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………
(ii) What was the solubility of each salt at 650C? (1 mark)
(iii) 100g of a saturated solution of potassium nitrate at 700C was cooled to 200C. What mass of the crystals will be crystallized? (2 marks)
(b) Study the flow chart below and answer the questions that follow.
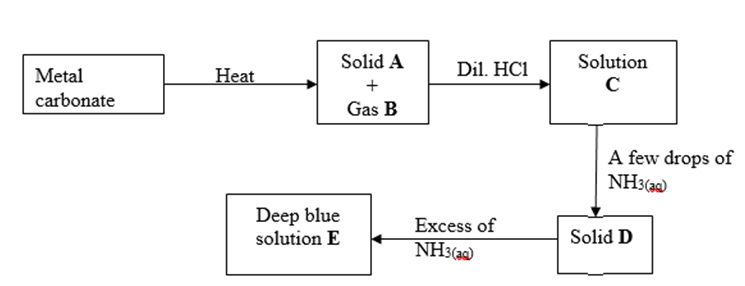
(i) Write an equation for the formation of solid A and gas B. (1 mark)
……………………………………………………………………………………………………………………………
(ii) Name;
Solution C – ………………………………………………….. (1 mark)
Solid D – ………………………………………………….. (1 mark)
(c) Write the formula of the complex ion in solution E. (1 mark)
……………………………………………………………………………………………………………………………
Chemistry Prediction 2022 Kapsabet
4. Study the flow chart below and answer the questions that follow.
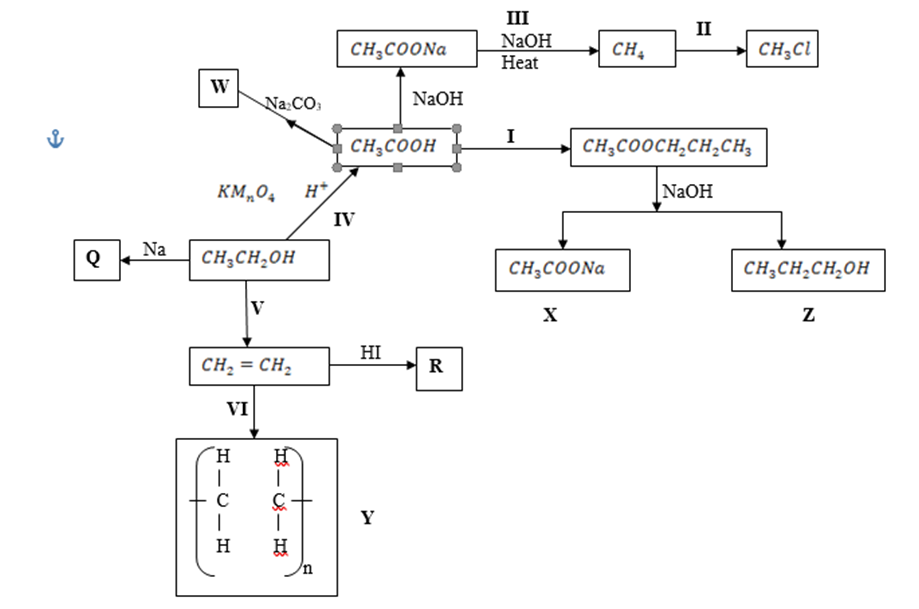
| NaOHHeat |
(a) Name substance. (3 marks) X – ………………………………………………………………….
Q – ………………………………………………………………….
R – ………………………………………………………………….
(b) Write down an equation for the reaction represented by step III. (1 mark)
……………………………………………………………………………………………………………………………
(c) What are the conditions and reagent required for steps?
(i) I (2 marks)
Reagent – …………………………………………………………………………………..
Condition – …………………………………………………………………………………..
(ii) IV (2 marks)
Reagent – …………………………………………………………………………………..
Condition – …………………………………………………………………………………..
(b) Name the process represented by: (4 marks)
I – ……………………………………………………………………….
II – ……………………………………………………………………….
IV – ……………………………………………………………………….
V – ……………………………………………………………………….
5. I. Study the scheme below and answer the questions that follow.
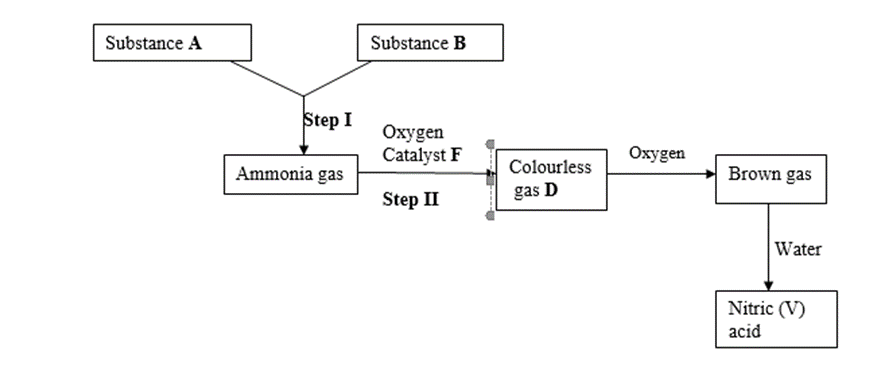
(a) Identify substances. (3 marks)
A – …………………………………………………………………..
B – …………………………………………………………………..
D – …………………………………………………………………..
(b) State the catalyst necessary for; (2 marks)
Step I – …………………………………………………………………………………………
Step II – …………………………………………………………………………………………
(c) Write an equation for the reaction taking place in step II. (1 mark)
………………………………………………………………………………………………………………………….
(d) Write two balanced chemical equations for the reaction between chlorine
gas and;
(i) Hot and concentrated sodium hydroxide. (1 mark)
………………………………………………………………………………………………………………….
(ii) Dilute and cold sodium hydroxide. (1 mark)
…………………………………………………………………………………………………………………
II. The diagram below shows an experiment in which the Lead (II) nitrate crystals are heated.
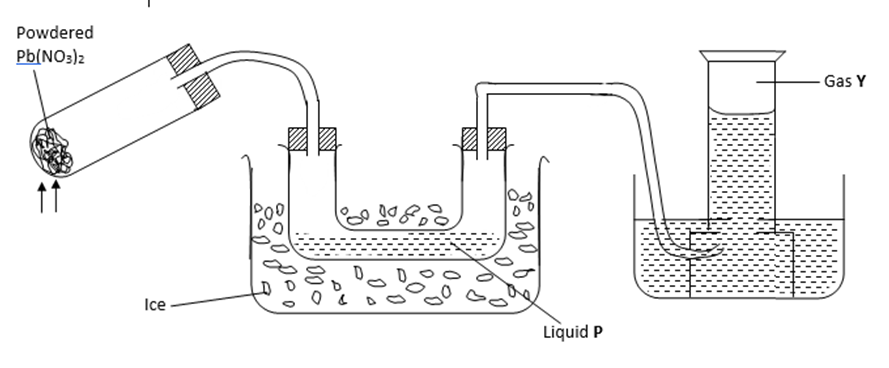
(a) Name; (2 marks)
(i) Liquid P – ………………………………………………………………………..
(ii) Gas Y – ………………………………………………………………………..
(b) Write a balanced chemical equation for the decomposition of Lead (II) nitrate. (1 mark)
…………………………………………………………………………………………………………………………
(c) Explain how you can distinguish between nitrogen (II) oxide and nitrogen (I) oxide.
(2 marks)
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………………………………
6. I. Study the standard electrode potentials given below and answer the questions that
follow.

(a) Identify the strongest:
(i) Reducing agent ……………………….. (1 mark)
(ii) Oxidizing agent ……………………….. (1 mark)
(b) Calculate the e.m.f of a cell made of G and M. (2 marks)
(c) Write the cell representation for the above cell in (b). (1 mark)
(d) Draw a cell diagram for the cell in (b) above. (2 marks)
(e) Write the cell reaction for the drawn cell diagram in (d) above. (1 mark)
II. Electrolysis of aqueous solution of metal M resulted in the deposition of 1.07g of metal upon passage of a current of 1.32 amperes for 75 minutes.
(M = 52, 1F = 96500C)
(i) Calculate the quantity of electricity passed through the cell. (1 mark)
(ii) Calculate the charge on the metal ion. (3 marks)
7. Extraction of iron involves two main processes, smelting and refining. Below is the blast furnace which is used to smelt iron from its ore.
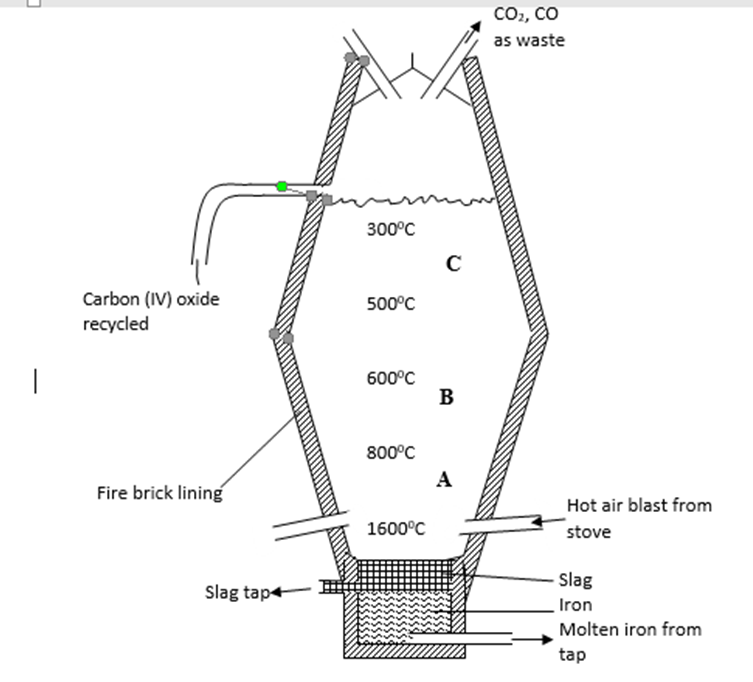
(a) (i) What does the word smelt mean? (1 mark)
…………………………………………………………………………………………………………………………….…………………………………………………………………………………………………………………………….
(ii) Name the reducing agent in the process. (1 mark)
……………………………………………………………………………………………………………………………. …………………………………………………………………………………………………………………………….
(iii) What is the role of the hot air blast in the process? (2 marks)
…………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………….
(b) Write equations for the reactions that take place at the region marked A, B and C. (3 marks)
A – ……………………………………………………………………………………………………….
B – ……………………………………………………………………………………………………….
C – ……………………………………………………………………………………………………….
(c) What is the purpose of limestone in the extraction process? (1 mark)
…………………………………………………………………………………………………………………………….
(f) Write equations to show how impurities are removed from the ore.
(3 marks)
…………………………………………………………………………………………………………………………….…………………………………………………………………………………………………………………………….…………………………………………………………………………………………………………………………….