1. An element K has atomic number 20 while element M has atomic number 8.
a) Write the electronic configuration for K and M
K
………………………………………………………………………………………….. 1mark
M
…………………………………………………………………………………….……. 1mark
b) Write the symbol of the most stable ion of K and M
K
…………………………………………………………………………………………. 1mark
M
…………………………………………………………………………………………. 1mark
2. Molten Lead (II) bromide is electrolyzed using carbon electrodes. Write the half equations of the reactions that occur at the anode and the cathode.
a) Anode
…………………………………………………………………………………….…… 1mark
b) Cathode
……………………………………………………………………….………………… 1mark
3. Explain why the conductivity of metals decreases with increase in temperature. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
4. Three metal oxides XO, YO, and ZO are heated with powdered metal Y. Hot powdered Y will remove oxygen from XO but not from ZO. Arrange the metals in order of reactivity, starting with the most reactive. 1mark
…………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
5. Some sodium chloride was found to be contaminated with copper (II) oxide. Describe how a sample of sodium chloride can be separated from the mixture. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
6. Hot platinum wire was lowered into a flask containing concentrated ammonia solution as shown below.

State and explain the observations made. 3marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
7. The set up below represents the apparatus that may be used to separate a mixture of two miscible liquids C and D whose boiling points are 800C and 1100C.
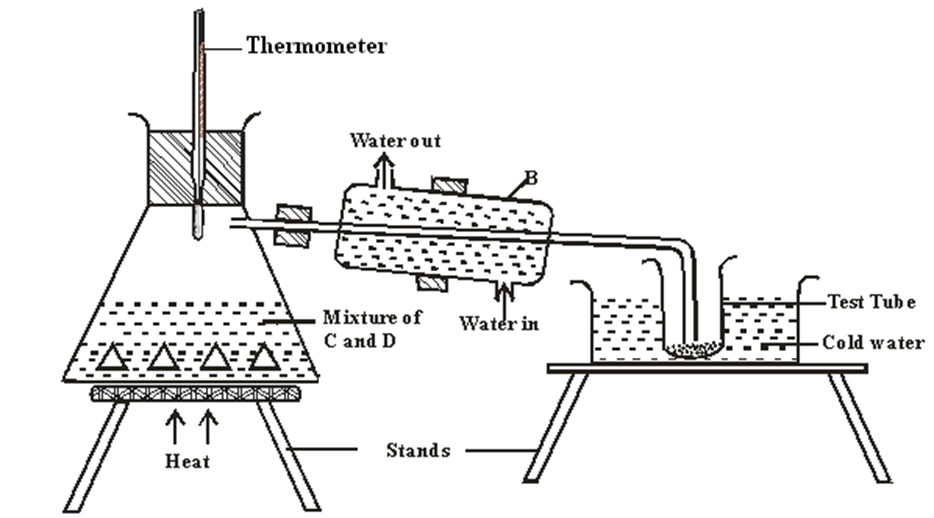
a) Name B
………………………………………………………………………………….. 1mark
b) What is the purpose of the thermometer 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
c) Which liquid was collected in the test tube? 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
8. Draw a dot (.) and cross (x) diagram to show bonding in carbon (II) oxide. 2marks
9. Ammonium nitrate was gently heated and the products collected as shown in the diagram.
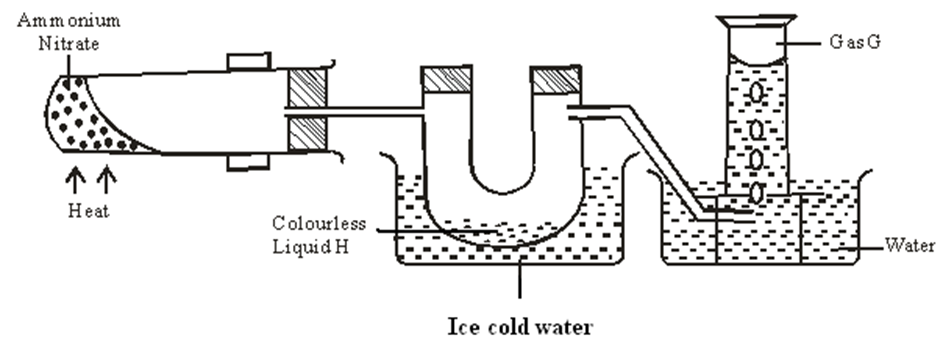
a) Identify:
i. Colourless liquid H
………………………………………………………………………………………… 1mark
ii. Gas G
………………………………………………………………………………………… 1mark
b) Describe one physical and one chemical test that can be used to identify gas G. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
10. Air was passed through several reagents as shown in the flow chart below.
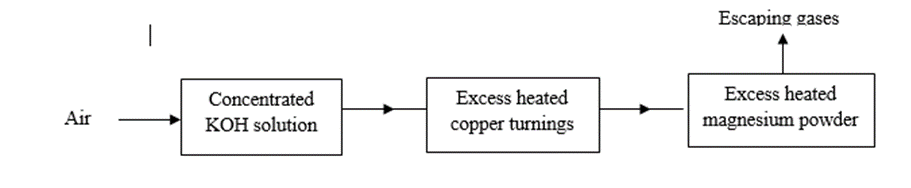
a) What is the purpose of concentrated potassium hydroxide solution? 1mark
……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
b) Write an equation for the reaction which takes place in the chamber with magnesium powder. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………..
c) Name one gas which escapes from the chamber containing magnesium powder.
………………………………………………………………………………………………………………………..
Give a reason for your answer 2marks
……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
11. Name the following substances.
a) CH2 CH CH2 CH3 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
b) CH3 CH CH CH2 CH3 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
12. The diagram below shows the acidic and basic oxides fit into the general family of oxides.

a) State the name given to the type of oxide that would be placed in the shaded area. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
b) Give the name of any oxide that would be placed in the shaded area. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
13. Study the information in the table below and answer the questions that follow. The letters do not represent the actual symbols of the elements.
| Substance | Solubility in water | Electrical conductivity | |
| Solid | Molten | ||
| A | Insoluble | Good | Good |
| B | Soluble | Poor | Good |
| C | Insoluble | Poor | Poor |
i) Which of the substances is highly likely to be sodium chloride? Explain 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
ii) What type of bond exists in substance A? 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
iii) State a possible structure in substance C? 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
14. Laboratory results showed the composition of a compound to be 58.81% barium, 13.72%, sulphur and 27.47% Oxygen. Calculate the empirical formula of the compound. Ba=137, S = 32, O = 16. 2marks
15. The diagram below shows a wooden splint that was placed horizontally across the middle part of a non-luminous flame.
a) Explain the observation made 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
b) Explain why non-luminous flame is preferred for heating than the luminous flame. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
16. 200cm3 of oxygen gas took 60 seconds to diffuse through a porous plug. Determine the time taken by 300cm3 of sulphur (IV) oxide to diffuse through the same plug under the same conditions.
(O=16, S = 32) 3marks
17. Explain why?
i) Both methane and diamond are covalently bonded. Methane is a gas but diamond is a solid with very high melting point. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
ii) Ammonia is dissolved in water using an inverted funnel. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
18. Explain giving reasons why?
a) Sulphuric acid is not used with marble in the preparation of carbon (IV) oxide 2marks
…………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………..…………………..
b) Water cannot be used to distinguish oil fire. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
19. A gas occupies 4dm3 at -230C and 152 mmHg. At what pressure will its volume be halved, if the temperature then is 2270C.? 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
20. a) Sodium, Magnesium and Aluminium are elements in the periodic table. Explain why aluminium has a higher melting and boiling point than sodium and magnesium. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
b) The ionization energy of an atom is strongly influenced by three atomic parameters. State two of these parameters. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
21. 15cm3 of a solution containing 2.88g/dm3 of an alkali XOH completely reacts with 20cm3 of 0.045M sulphuric acid. Calculate the molarity and relative atomic mass of X present in the alkali. 3marks
22. Describe how a solid sample of calcium sulphate can be prepared using the following reagents; dilute nitric (v)acid, dilute sulphuric (vi) acid and solid calcium carbonate 4marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
CHEMISTRY Paper 1 Prediction 2022 Kapsabet
23. Crude oil is the main source of organic compounds such as hydrocarbons. The hydrocarbons in the crude oil have to be separated.
a) Name two important hydrocarbons obtained from crude oil. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
b) Give the uses of the two hydrocarbons named in (a) above. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
24. A hydrocarbon Q was found to decolourise potassium manganate (vii) solution. When two moles of Q were burnt completely six moles of carbon (iv) oxide and six moles of water were formed.
a) Write the structural formula of Q. 1mark
……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
b) Name the homologous series to which Q belongs 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
25. Dilute sulphuric acid was added to a compound X, of magnesium. The solid reacted with the acid to form a colourless solution, Y and a colourless gas Z which formed a white precipitate when bubbled through lime water.
Name:-
(i) Compound X 1mark
………………………………………………………………………………………………………………
(ii) Solution Y 1mark
………………………………………………………………………………………………………………
(iii) Colourless gas Z 1mark
………………………………………………………………………………………………………………
26. When dry hydrogen gas passed over heated Lead (II) oxide in combustion tube, a grey solid was formed.

a) Identify the grey solid. 1mark
…………………………………………………………………………………………………………….
b) Write the equation of the reaction taking place in the combustion tube. 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
c) Write the equation involving the blue flame. 2marks
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
27. What do (C F C’ S) mean? 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
28.
a) What is meant by the term allotrophy? 1mark
………………………………………………………………………………………………………………………………………………………………………………………………………………………………
b) Explain in terms of structure and bonding why graphite is soft with greasy feeling. 2marks