- State four preventive measure of drug –abuse.(4mks)
- a.State under which conditions are the following flame formed in the laboratory. 2mks
- Luminous…………………………………………………………………..
- Non- luminous…………………………………………………………..
b.In an experiment, a form one student at Kiranja Secondary School placed an end of narrow glass tubing in the inner core of non-luminous flame and lit at the tip of the glass tubing as shown below .

State and explain the observation made at the tip of the glass tubing. (2mks)
- State one application for each of the following methods of separating mixtures.
- Filtration (1mk)
- Fractional distillation(1mk)
- Solvent extraction.(1mk)
- a.State two ways for determining the purity of substance.(2mks)
–
–
b.The diagram below represents heat curves of a pure surface of solid .Study it and answer the questions that follows.
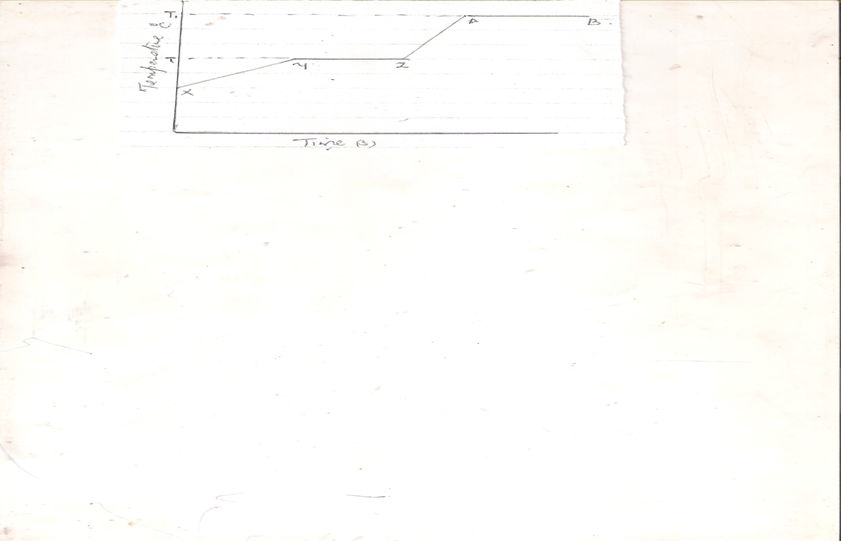
- Region YZ and AB have one thing in common .State it and explain.(2mks)
ii.State the physical state of substance at the following region
XY. (2mks)
ZA (1mk)
- Define the following terms.
- Solution 1mks
- Saturated solution 1mks
- The following is a set up to prepare oxygen gas .Study it and answer the questions that follows.
- Identify the mistake made when setting up the apparatus. (2mks)
- If the mistake was corrected, complete the diagram showing how to collect dry oxygen gas. (3mks)
- Identify liquid L . ( 1mk)
- Write word equation for reaction at round- bottomed flask. (2mks
- State two physical properties of oxygen.2mks)
- Identify the following ways of collecting gases and state the reason.
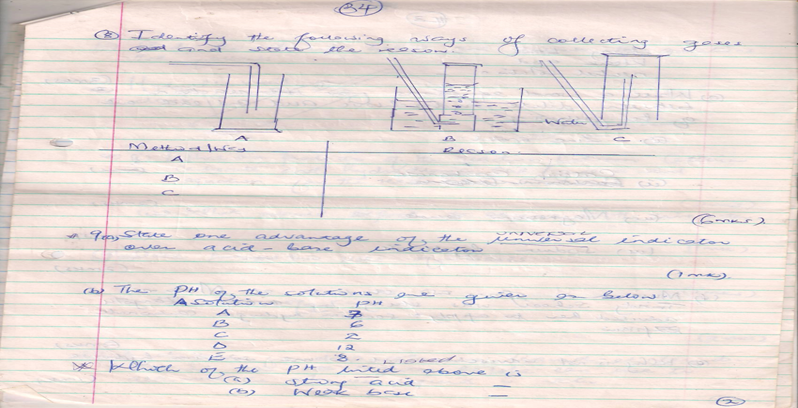
Method/Way Reason
A
B
C
(3mks)
- a. State one advantage of the universal indicator over acid- base indicator.(1mk)
b.The pH of the solutions are given as below
Solution pH
A 7
B 6
C 2
D 12
E 8
Which of the pH listed above is (5mks)
- Strong acid –
- Weak base –
- Strong base-
- Weak acid-
- Neutral solution-
c.Write a word equations for the reaction between dilute hydrochloric acid and each one of the following
i. Zinc metal (2mks)
ii. Calcium carbonate (2mks)
iii.Magnesium Oxide(2mks)
IV.Sodium hydroxide (2mks)
d.Miriam a form two student at Mutuma Trinity School was stung by wasp.Kanyotu advised her to apply sodium hydrogen carbonate .Explain. (2mks
e.Why is not advisable not to use sodium hydroxide.(1mk)
f.Study the set-up below and answer the questions that follows.
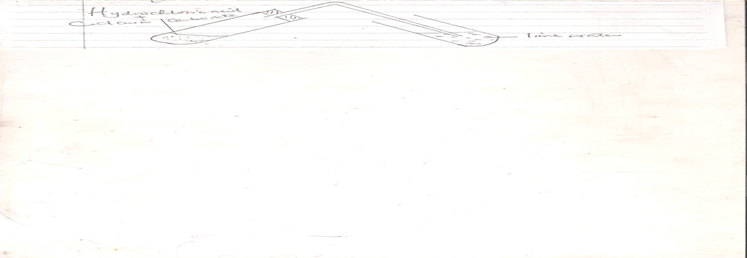
- What are the products of the reaction between calcium carbonate and hydrochloric acid(give a word equation for the reaction). (2mks)
- What would expect to observe in the limewater? (1mk)
g.i)State two uses of bases.(2mk)
ii)Two uses of acids. (2mks)
- A student found a colourless liquid at laboratory. Describe two reagents he/she can use to determine the colourless liquid is water.(2mks)
- a.State two differences between permanent change and temporary physical changes.
Permanent change Temporary physical changes
(4mks)
b.Study the following chemical equations (2mks)
heat
- Zinc oxide zinc oxide
(white) yellow
- Potassium Potassium + Manganeese (iv) + oxygen
Manganate(vii) Manganete(vi) oxide
- Hydrated copper(ii) Copper (ii) oxide + Water
Oxide
Identify the changes in (3mks)
Reaction I
Reaction II
Reaction III
- The diagram below represents a paper chromatogram for three brands of juices suspected to contain banned food colourings.

The results showed the presence of banned food colourings in L and M.One the same diagram.
- Give the spots which show the banned food colourings.(2mks)
- Show solvent front.(1mk)
- State two applications of chromatography.(2mks)
- The apparatus below were used to determine the volume of oxygen in air. About 200cm3 of air were passed repeatedly and slowly from syringe A to syringe B, over heated copper turnings as shown in the diagram.
Copper turnings
After sometime the volume of air syringe A was 160cm3 and syringe B 0cm3.
- Calculate the percentage of oxygen in the initial sample of air.(2mks)
- Write down a word equation for the reaction that took place in the combustion tube.(1mk)
- What are possible sources of error in the experiment.(2mks)
- Explain why the air is passed slowly and repeatedly.(1mk)