BOYLE’S LAW QUESTIONS AND ANSWERS.
‘CHARLE’S LAW’ KCSE Prediction Questions and Answers
Chemistry Topical Questions and Answers for the Topic Pressure Laws
State the pressure law for an ideal gas (1mk)
ü The pressure of a fixed mass of a gas is directly proportional to the absolute temperature(Kelvin) at a constant volume.
- A gas is enclosed in a glass container and the container is heated. Explain why the pressure of the gas increases. (1mk)
ü Raising the temperature increases the average speed of particles so they collide more vigorously and more frequently with container walls. If the volume does not increase the pressure must rise.
- Explain why gas cylinders are likely to expand incase of a fire out break(2mk)
answer : When a gas is heated, Temperature of a gas rises, its particles move faster and exert a larger force on the wall of the cylinder.
- Using Kinetic theory of Gases, explain how the rise in temperature of a gas causes rise in the pressure of a gas if the volume is kept constant.(3mk)
- Raising the temperature increases the average speed of particles so they collide more vigorously and more frequently with container walls.
- If the volume does not increase, pressure increases.
- Raising the temperature increases the average speed of particles so they collide more vigorously and more frequently with container walls.
- A house in which a cylinder containing cooking gas is kept unfortunately catches fire. The cylinder explodes. Give a reason for the explosion.
Ø From pressure law, when a gas is heated due to fire Temperature increases which led to increase in pressure at constant volume, when pressure increases it can led to explosion.
- Define the absolute zero temperature (1mk)
Ø Absolute zero temperature refers to the lowest temperature a gas can fall to. It is -2730celcius (0 Kelvin).
- The diagram below shows a set up that a student used to investigate the pressure law.
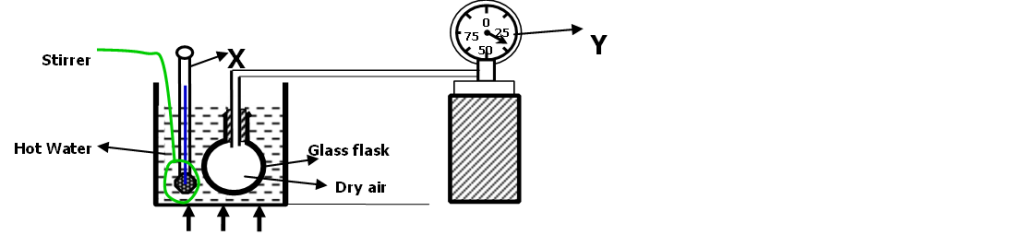
a) Name the parts (2mks)
X ……………………Thermometer……………………………………………….. Y ……………………Pressure gauge……………………………………………..
b) What are the functions of (2mks)
(i) the stirrer
- To stir the water for uniform distribution of heat (ii) Part Y.
- To measure pressure
c) At what Kelvin temperature will the pressure of the air theoretically be zero.
(1mk)
Ø -2730C =0 K
d) What name is given to this temperature?
Ø Absolute zero temperature
(i) State the measurements that should be taken in the experiment(3mk) a) Temperature
b) Pressure
(ii) Explain how the measurement in (i) above may be used to verify
Pressure law (3mk)
- The initial temperature and pressure reading are taken and recorded § The water bath is heated gently and some more pairs of pressure and temperature readings are taken and recorded at suitable temperature intervals
- A graph of pressure against temperature is plotted.
- It is a straight line with positive gradient.
- This shows that the pressure is directly proportional to absolute temperature.
(iii) Name one limitation of the gas laws. (1mk)
Real gases liquefy before the volume of the gas reduces to zero.
8. A gas is put into a container of fixed volume at a pressure of 2.1 x 105 Nm-2 and temperature 270C.The gas is then heated to a temperature of 3270C. Determine the new pressure.

At the start of the journey, the temperature and pressure of inside a car tyre were 170c and 300 Pa