Get the Answers here Form 4 End Term 1 Exams 2023 Questions and Answers
. The grid below represents part of the periodic table. Study it and answer the questions that
follow.
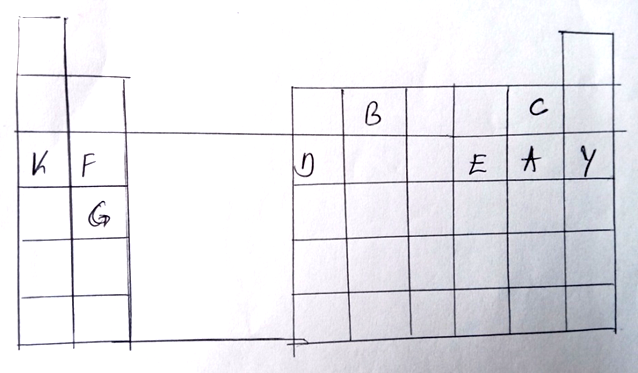
(a) Identity the family name to which element F and G belong. (1 mk)
Alkaline earth metals
(b) Name the type of bond formed when a and F belong. (1 mk)
Ionic bond
(c) Write the formula of the oxide formed when D reacts with oxygen. (1 mk)
D2O3
(d) What type of oxide is formed in (c) above. (1 mk)
(e) Compare the atomic radii of F and D. Explain. (2 mks)
– Has a smaller atomic radius than F because D has more protons hence stronger
to F.
(f) Element F burns in air to form two products. Write two equations of the two products
formed. (3 mks)
2F(s)
) F3N2(s)
(g) Stat e two uses of element K and its compounds. (2 mks)
– K cyanide is used in the extraction of gold
– Mixture of K and potassium is used as nuclear coolant.
2. (a) Name the following organic compounds.
(i) CH3CH2CH(Br)CH3
2, 3 – dibromo – 2 – chloropentane
(ii) CH3-CH2CH2-CH2-C-OH
Butanoic acid
(iii) CH2CHCH2CH(Br)CH3
4 – bromopent-i-ene
(b) Study the flow chart below and answer the questions that follow.
(a) 50cm3 of 1M copper (II) Sulphate solution was placed in a 100cm3 plastic beaker. The
temperature of the solution was measured. Excess metal A powder was added to the
solution, the mixture stirred and the maximum temperature was repeated using powder of
metal B and C. The results obtained are given in the table below.
| A | B | C | ||
| Maximum temperature oC | 2.63 | 31.7 | 22.0 | |
| Initial temperature (oC) | 22.0 | 22.0 | 22.0 |
(i) Arrange the metal A, B, C and Copper in order of reactivity starting with the least
reactive. Give reasons for the order. (3 marks)
C, Copper, A, B.
B is the most reactive because it has highest T.
C is the least reactive because it cannot displace ions of copper from solution.
A is more reactive than Copper because it displaces Cu2+ from solutions.
(ii) Other than temperature change, state one other observation that was made when the
most reactive metal was added to the copper (II) Sulphate solution. (1 mk)
– Blue colour of the solution fades /disappeared
– Black deposit is formed.
(b) The Standard enthalpy change of formation of methanol is -239Kjmol-1
(i) Write the thermal chemical equation for the standard enthalpy change of formation of
methanol. (1 mk)
C(s) + 2H2(g) + ½ O2(g) H3OH(g)
(ii) Use the following data to calculate the enthalpy change for the manufacture of methanol
from carbon (II) oxide and hydrogen. (3 mks)
CO(g) + ½ O2(g) CO2; Hɵ = -283Kj/mol
H2(g) + ½ O2(g) H2O(l); = Hɵ = -286Kj/mol
CH3OH + CO2(g) + 2H2O; Hɵ = -715Kj/mol
C + 2H2 + ½ O2 CH3OH
H= -283Kj/mol
O2 O2 O2
CO2 + H2O
σCH3OH = HcC + HcH2 – HcCH3OH
= -283 + 2(-286) – (-715)
= -283 – 576 + 715
= -859 + 715
f CH3OH = – 144Kj/mol
(c) Study the information given in the table below and answer the questions that follow.
| Bond | Bond energy (Kjmol-1 |
| C – H Cl – Cl C – Cl H – Cl | 414 244 326 431 |
Calculate the enthalpy change for the reaction. (3 mks)
CH4(g) + Cl2(g) CH3Cl(g) + HCl(g)
H H
│ │
H ─C─H + Cl─H H─C─Cl + H─Cl
│ │
H H
Bond breaking energy – Bond formation energy
BBE – BFE
4(414) + 244 = 3 (414 + 326 + 431
(1900 – 1999) = -99Kj
= -99Kj
4. Carbon IV oxide is produced when solid X is heated strongly. It can also be prepared by adding
dilute hydrochloric acid to solid X. The reaction between X and dilute Sulphuric acid, however
gradually slows down and stops.
(a) (i) Name solid X. (1 mk)
Calcium carbonate
(ii) Write an ionic equation for the reaction of X and acid. (1 mk)
CO32-(s) + 2H+(aq) CO2(g) + H2O(l)
(b) A gas jar full of Carbon (IV) oxide was inverted over burning candle.
(i) State the observations made. (1 mk)
Candle is immediately extinguished
(ii) What two properties of carbon (IV) oxide does this observation illustrate. (2 mks)
– It does not support combustion and it is denser than air since it is poured
downwards from gas jar.
(iii) Name a practical everyday use of this property of carbon (IV) oxide. (1 mk)
As a fire extinguisher
(c) The flow diagram below shows some reactions of calcium compounds.
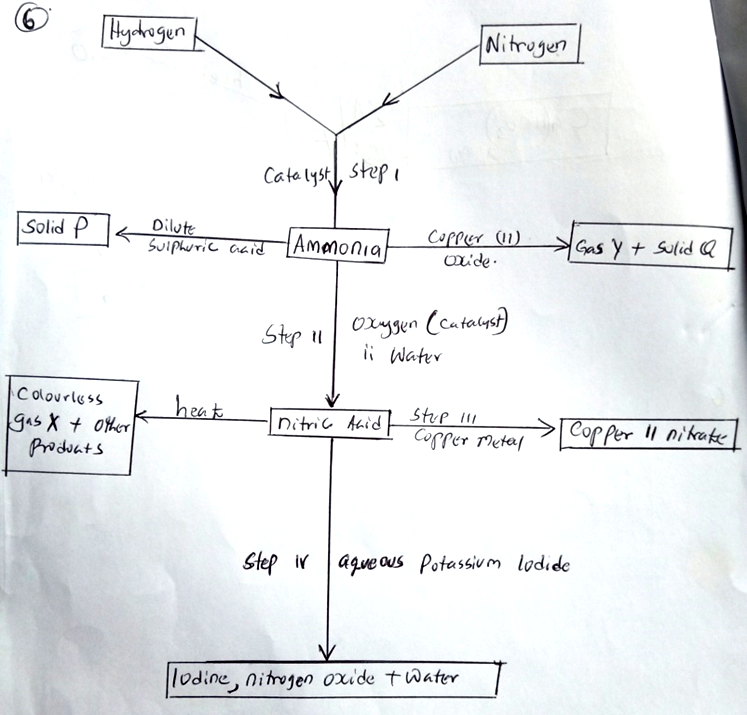
(i) Give one condition other than the of a catalyst that would favour the reaction in step I. (1 mk)
High pressure
(ii) Name the catalysts used in step I and II. (2 mks)
Step I – finely divided iron
Step II – Vanadium V oxide/platinum
(iii) Name substances P, Q, X and Y . (2 mks)
P – ammonium sulplhate
Q – Copper metal
X – Oxygen
Y – nitrogen gas
(iv) Write equations for the reactions that takes kplace in step II. (3 mks)
4NH3(g) + 5O2(g) 4NO(g) + 6H2O(l)
2NO(s) + O2(g) 2NO2
4NO2(g) + 2H2O(g) + O2(g) HNO3(aq)
(v) Name the oxidizing agent for the reaction that takes place in step IV. (1 mk)
Nitric acid
(vi) Why is a concentrated nitric acid transported on aluminium container and not copper? (1 mk)
Concentrated nitric acid with copper oxidizes it to Copper(II) nitrate, while aluminium forms layer of aluminium oxide which is a passive and stops any further action by the acid.
7. Use standard electric potentials for elements A, B, C, D and F given below to answer the
questions that follow.
Eɵ (volts)
A2+(aq) + 2e- A(s) -2.90
B2+(aq) + 2e- B(s) -2.38
C+(aq) + 2e- ½ C(g) -0.00
D2+(aq) + 2e- D(s) +0.34
½ F2(g) + e- F–(aq) +2.87
(i) Which element is likely to be hydrogen? Give a reason for your answer. (2 mks)
C+/C2 = hydrogen is used as the reference electrode
Eɵ value is 0.00/standard electrode potential
(ii) What is the Eɵ value of the strongest reducing. (1 mk)
Eɵ = -2.90V
(iii) In the space provided, draw a labeled diagram of the electrochemical cell that would be
obtained when half-cells of elements B and D are combined. (3 mks)
(iv) Calculate the Eɵ value of the strongest reducing agent. (2 mks)
2.38 + 0.34 = 2.72
0.34 –(-2.38) = +2.72
(0.34 + 2.38) = +2.72V
(b) During the electrolysis of aqueous copper II Sulphate using copper electrodes, al current of
0.2 amperes was passed through the cell for 5 hours.
(i) Write an ionic equation for the reaction that took place at the anode. (1 mk)
Cu(s) Cu2+(aq) + 2e-
or
Cu(s) Cu2+(aq)
(ii) Determine the change in mass of the anode which occurred as a result of the electrolysis
process. (C.u = 63.5, 1 Faraday = 96,500 coulombs) (2 mks)