Get The Marking Schemes Here
1. Element A has atomic mass 23 and element B has atomic mass 7 and also have 12neutorns and
4 neutrons respectively.
(a) Write the electronic arrangement of A and B (2mks)
(b) Which element has higher ionization energy? Explain (2mks)
2.The number of protons, neutrons and electrons in atoms A to F are given in the table below
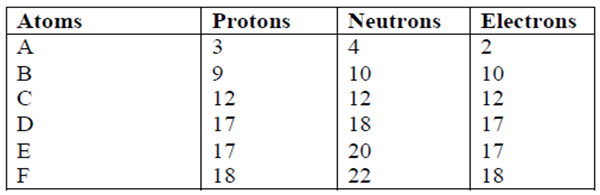
the letters do not represent the actual symbol of the elements:-
(a) Choose from the table the letters that represent:
(i) An atom of a metal (1mk)
(ii) A neutral atom of a non-metal (1mk)
(iii) An atom of a noble gas (1mk)
(iv) A pair of isotopes (1mk)
(v) A cation (1mk)
3.The grid below represents part of the periodic table. Study it and answer the questions that follow:
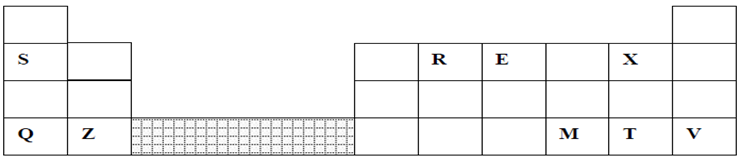
(a) (i) Identify the element that gains electrons most readily (1mk)
(ii) Which of the metal is most reactive? Explain (2mks)
(iii) What name is given to the family of elements to which elements X and T belong? (1mk)
(iv) Explain why:-
(I) Ionic radius of Q is larger than that of M (1mk)
(II) Atomic radius of Q is greater than that of S (1mk)
(v) Which of the element in the table does not have the ability to form an ionic or covalent
bond? Explain (2mks)
(vi) Give the formula of the compound formed between R and Z (2mks)
4.The structure of ammonium ion is shown below;
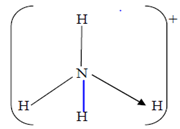
a)Name the type of bond represented in the diagram by N H (1mk)
b). Study the table below and answer the questions that follow:-
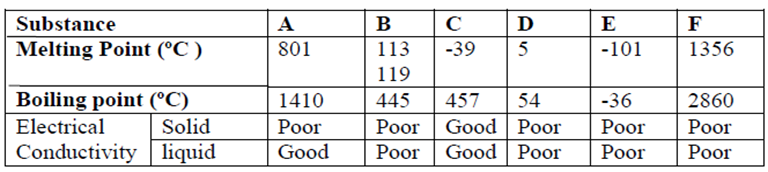
I Identify with reasons the substances that:
(i) Have a metallic structure (1½mk)
(ii) Have a molecular structure and exist in the liquid state at room temperature and pressure (1½mk)
(iii) Suggest a reason why substance B has two melting points (1mks)
(iv) Substances A and C conduct electric current in the liquid state. State how the two substances
differ as conductors of electric current (2mks)
5.In an experiment, ammonium chloride was heated in test-tube. A moist red litmus paper
placed at the mouth of test first changed blue then red. Explain these observations:- (2mks)
6. a) Give the name of each of the processes described below which takes place when salts are
exposed to air for sometime:- (3mks)
i) Anhydrous copper sulphate becomes wet
ii) Magnesium chloride forms an aqueous solution
iii) Fresh crystals of sodium carbonate, Na2CO3.10H2O become covered with white powder
of formula Na2CO3.H2O
7. (a) Write an equation to show the effect of heat on the nitrate of:- (2mks)
(i) Potassium
(ii) Silver
8.You are provided with the following:- solid lead (II) nitrate, magnesium oxide powder,
dilute sulphuric (VI)acid and distilled water. Describe how you can prepare a dry sample
of lead (II) sulphate. (3mks)
9.Study the diagram below and use it to answer the questions that follow:-
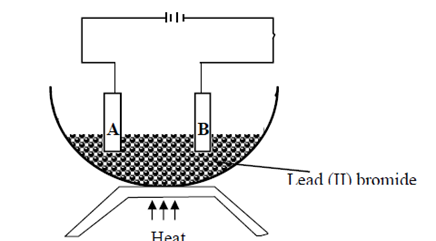
(a) Identify electrodes A and B (2mks)
(b) Name the product formed at the anode (1mk)
(c) Write the electrode half equation of reaction at electrode A (1mk)
10.Below is a simplified scheme of Solvay process. Study it and answer the questions that follow.
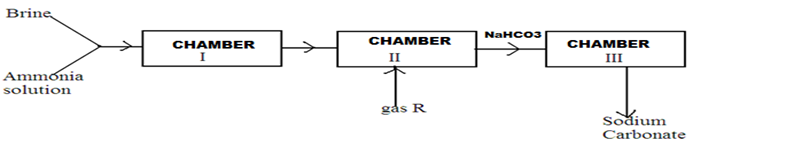
(a).Identify gas R. (1mk)
(b)Write an ionic equation for the process in chamber III. (1mk)
(c).Give two uses of sodium carbonate. (2mks)
11. The set up below was used to collect gas K, produced by the reaction between water and
calcium metal.
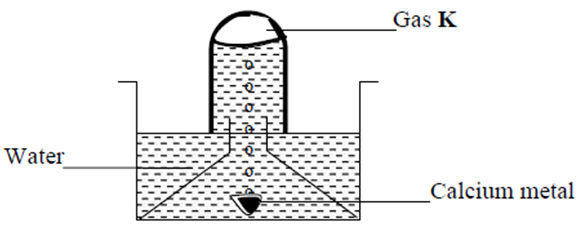
(a) Name gas K (1mk)
(b) At the end of the experiment, the solution in the beaker was found to be a weak base. Explain
why the solution is a weak base
12. Name the process which takes place when (2mks)
(a) Solid Carbon (Iv) Oxide (dry ice) changes directly into gas
(b) A red litmus paper turns white when dropped into chlorine water
13.A student left some crushed fruit mixture with water for some days. He found the mixture
had fermented. He concluded that the mixture was contaminated with water and ethanol with boiling point of 100oC and 78oC respectively. The set-up of apparatus below are used to separate
the mixture.
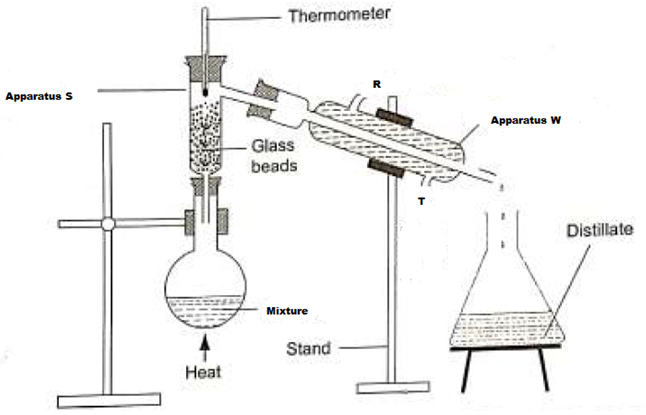
(i) Name the piece of apparatus labelled W (1mk)
(ii) What is the purpose of the thermometer in the set-up? (1mk)
iii) At which end of the apparatus W should tap water be connected? (1mk)
(iv) Which liquid was collected as the first distillate? Explain (1mk)
(v) What is the name given to the above method of separating mixture? (1mk)
(vi) State two applications of the above method of separating mixtures (1mk)
(vi) What properties of the mixture makes it possible for the component to be separated
by the above methods? (1mk)
14.The following diagram shows a paper chromatogram of substances A, B, C, and D which
are coloured
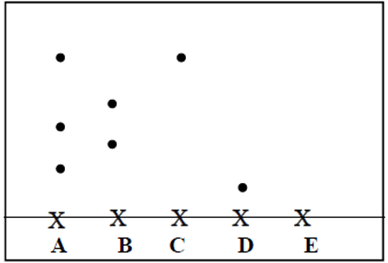
(a) Indicate the solvent front on the chromatogram (1mk)
(b) Which substance is pure? (1mk)
(c) Substance E is a mixture of C and D. Indicate its chromatogram in the diagram (1mk)
15.The diagram below shows students set-up for the preparation and collection of oxygen gas
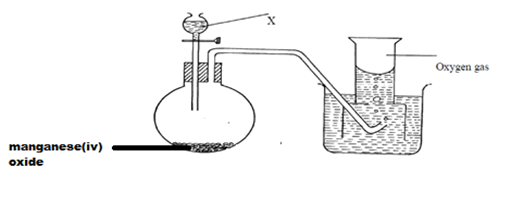
(a) Name substance X used (1mk)
(b) Write an equation to show the reaction of sodium peroxide with the substance named in (1mk)
15. A student set-up the experiment as shown below to collect a gas. The wet sand was heated before
heating zinc granules
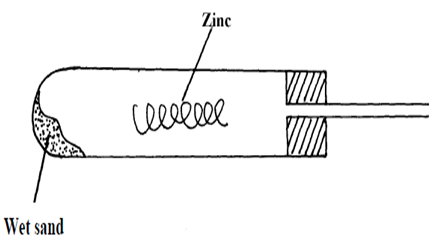
(a) Complete the diagram for the laboratory preparation of the gas (3mks)
(b) Why was it necessary to heat wet sand before heating Zinc granules? (1mks)
16. Below are PH values of some solutions.
| Solution | A | B | C | D |
| PH | 6.7 | 13.0 | 2.1 | 7.0 |
i) Which solution is likely to be
I. rain water (½ mark)
II. Sodium hydroxide (½ mark)
ii) Which substances will be formed when magnesium reacted with solution C?(1 mark)
17.An experiment was set up using chlorine water as shown below.
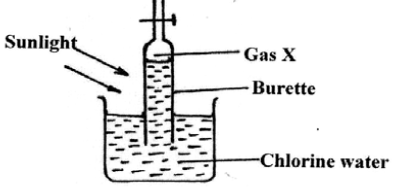
(i) Identify gas X. (1mk)
(ii) Write an equation for the production of gas X. (1mk)
(iii)State any TWO uses of chlorine gas. (2mks)
18.The set-up below was used to collect gas F produced by the reaction between sodium
peroxide and water.
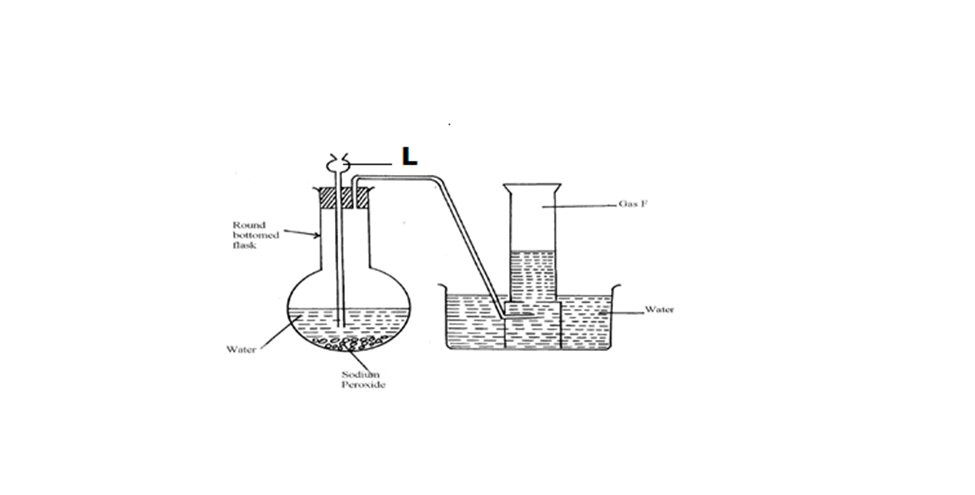
(i) Name gas F (1mk)
(ii) At the end of the experiment, the solution in the round bottomed flask was found to be
a strong base. Explain why this was so. (1mk)
(iii) Which property of gas F makes it be collected by the method used in the set-up? (1mk)
(iv) Give one industrial use of gas F (1mk)
19. Complete the following table to show the colour of the following indicators in acidic and basic solution (2mks)
| indicator | Colour in | |
| Acidic solution | Basic solution | |
| Phenolphthalein | ||
| Methyl orange | Yellow | |
| Litmus solution | Red |
20.Define the following terms (2mks)
i) Cation
ii)Isotopes
21. The diagram below represents an allotrope of carbon.
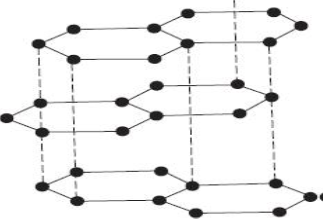
a) name the allotrope. (1mk)
b)Explain why:- (2mks)
(i) Its slipperly
(ii) Conducts an electric current