BOYLE’S LAW QUESTIONS AND ANSWERS.
‘PRESSURE LAW’ KCSE Prediction Questions and Answers
Chemistry Questions and Answers for the Topic Gas Laws
.
1. State Charles law for an ideal gas. (1mk)
ü The volume of a fixed mass of a gas is directly proportional to the absolute temperature(Kelvin) at constant pressure.
2. When an inflated balloon is placed in a refrigerator it is noted that its volume reduces.
Low temperature reduces the kinetic energy of molecules, and therefore the rate at which they collide with the walls of the container is reduces which results to reduction of volume.
3. Define absolute zero temperature for an ideal gas (1mk)
ü Absolute zero temperature refers to the lowest temperature a gas can fall to. It is -2730celcius (0 Kelvin).
4. The pressure of the air inside a car tyre increases if the car stands out in the sun for some time on a hot day. Explain the pressure increase in terms of the kinetic theory of gases. (3mk)
When the temperature of the air inside the tyre increase the kinetic energy of air particles increases and therefore the rate at which they collide with the walls of the tyre is increased – so the pressure increases.
5. Using the kinetic theory of gases, explain the behaviour of gas particles in accordance with Charles Law. (2mk)
- When a gas is heated the kinetic energy of its molecules increases.
- If the volume remains constant the pressure at the walls would increase due to a greater rate of change of momentum per unit time.
- Since Charles law is done at constant pressure, and then the volume increases.
- An index of sulphuric acid
- A thermometer
- A stirrer
- A meter scale (Ruler)
- Water bath and a source of heat
- If the volume remains constant the pressure at the walls would increase due to a greater rate of change of momentum per unit time.
- 7. You are provided with the following A uniform glass tube
Using a suitable diagram, explain how the above may be used to verify Charles’ law.
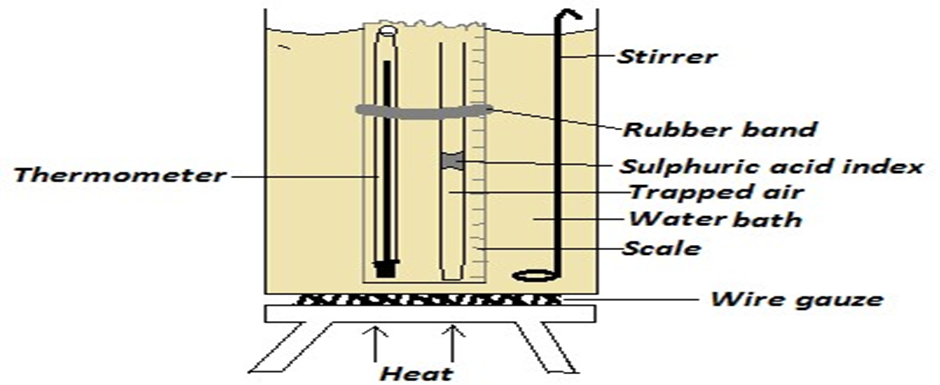
- The initial length of the air column is taken and recorded as well as the initial thermometer reading.
- The water bath is heated and new height (column) of air is taken and recorded with its corresponding temperature reading
- This is repeated several times at suitable temperature intervals to get several pairs of results
- A graph of volume (height, h (cm)) against absolute temperature is plotted. It is a straight line with positive gradient.
- This shows that the volume is directly proportional to absolute temperature.
‘CHARLE’S LAW’ KCSE Prediction Questions and Answers
The diagram in figure below shows an experiment to investigate the relationship between volume and temperature of a fixed mass of gas at constant pressure.
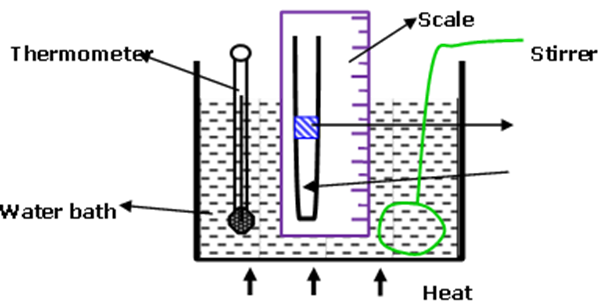
(a) Explain the function of:
(i) Concentrated sulphuric acid (2mk)
- Used as a pointer to volume of the gas on the scale
- Used as a drying agent for the air
- Used to trap air
- Used as a drying agent for the air
(ii) Stirrer (1mk)
To stir the water bath for uniform distribution of heat
(b) Which measurements are taken in the above experiment (2mk) Air column height which corresponds to volume
Temperature
- State law being investigated in the experiment above.(1mk) ü Charles’ law
- Describe how the experiment above is used to verify the law. (3mk)
- The initial length of the air column is taken and recorded as well as the initial thermometer reading.
- The water bath is heated and new height (column) of air is taken and recorded with its corresponding temperature reading
- This is repeated several times at suitable temperature intervals to get several pairs of results
- A graph of volume (height, h (cm) against absolute temperature is plotted.
- It is a straight line with positive gradient.
- This shows that the volume is directly proportional to absolute temperature.
(e) What physical property of the gas is kept constant in this experiment?
(1mk)
ü Pressure
(f) Why is the atmospheric pressure not taken into account in this experiment?
(1mk)
ü Atmospheric pressure is also constant.
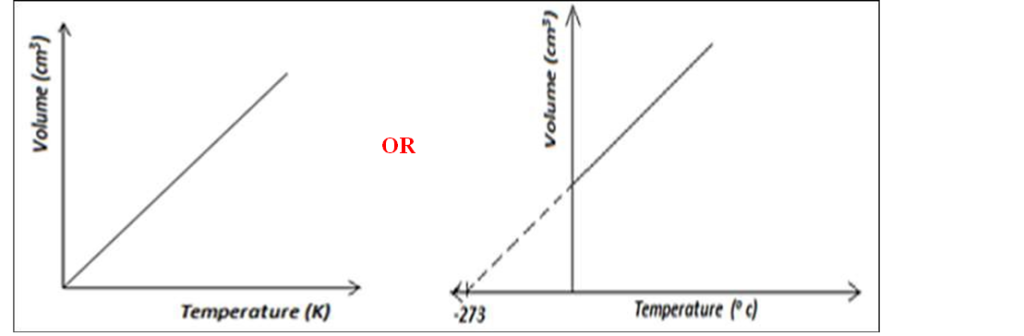
(g) On the grid shown in figure below sketch a graph of volume (cm3) against temperature (0C) for the experiment above. Clearly mark with the letter T the
absolute zero temperature. (2mk)

Sketch a graph of volume against absolute temperature for an ideal gas.(2mk)
- A gas has a volume of 20cm3 at 270C and normal atmospheric pressure.
Calculate the new volume of the gas if it is heated to 540C at the same pressure.

On a certain day when the temperature is 370c, the pressure in an open gas jar is 640mm of mercury. The jar is then sealed and cooled to the temperature of 170c. Calculate the final pressure.

A constant mass of hydrogen gas occupies a volume of 4.0cm3 at a pressure of 2.4 x 105 Pa and temperature of 288K. Find its volume at a pressure of 1.6 x 105 Pa when the temperature is 293K.
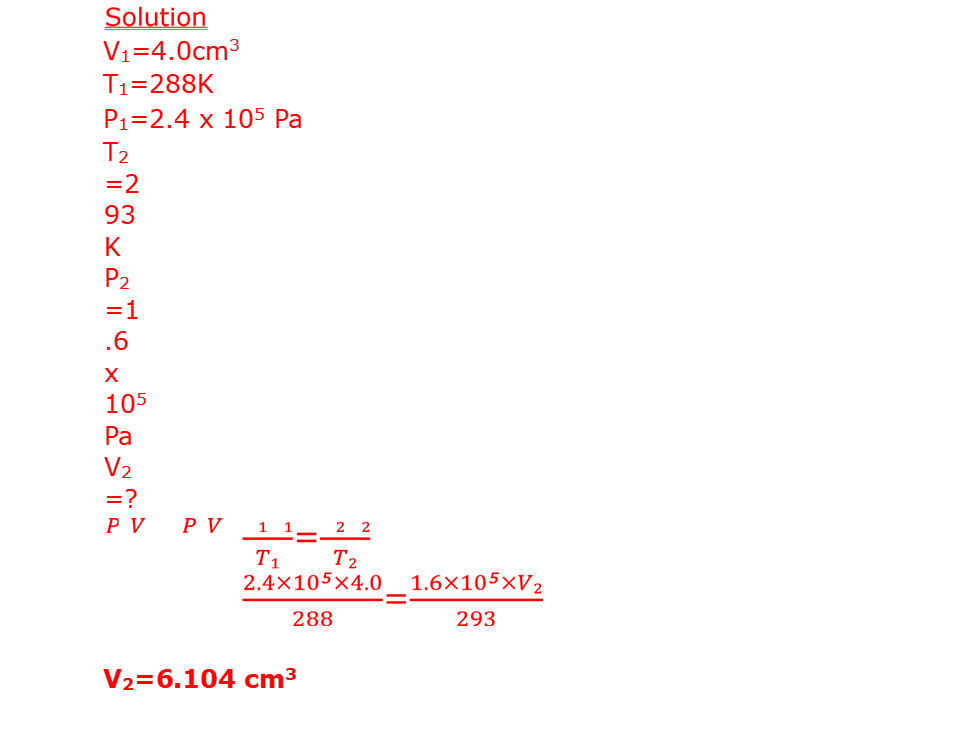
A balloon is filled with air to a volume of 200ml at a temperature of 293 K.
Determine the volume when the temperature rises to 353 k at the same pressure
(3mk)